Development of Vaccines and Drugs for CoVID – 19 Worldwide
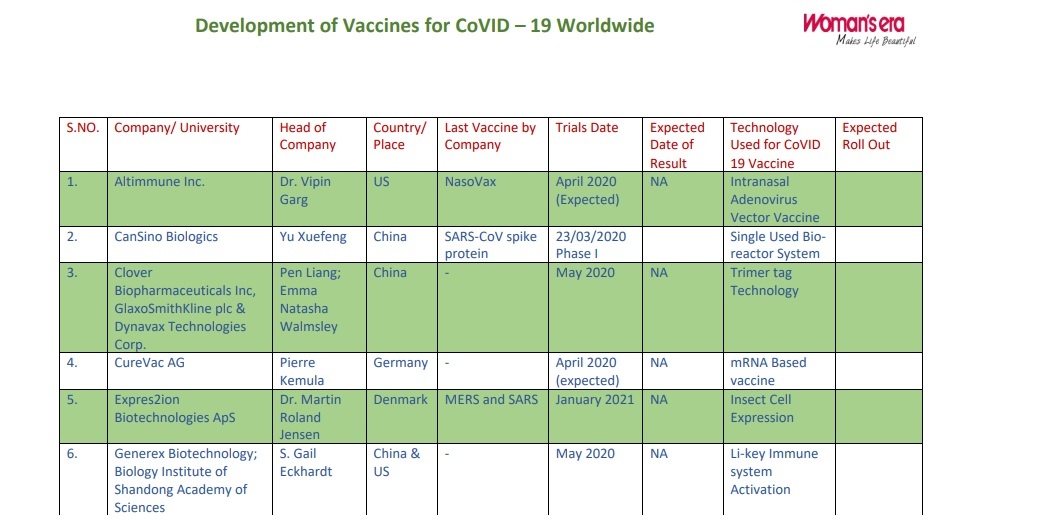
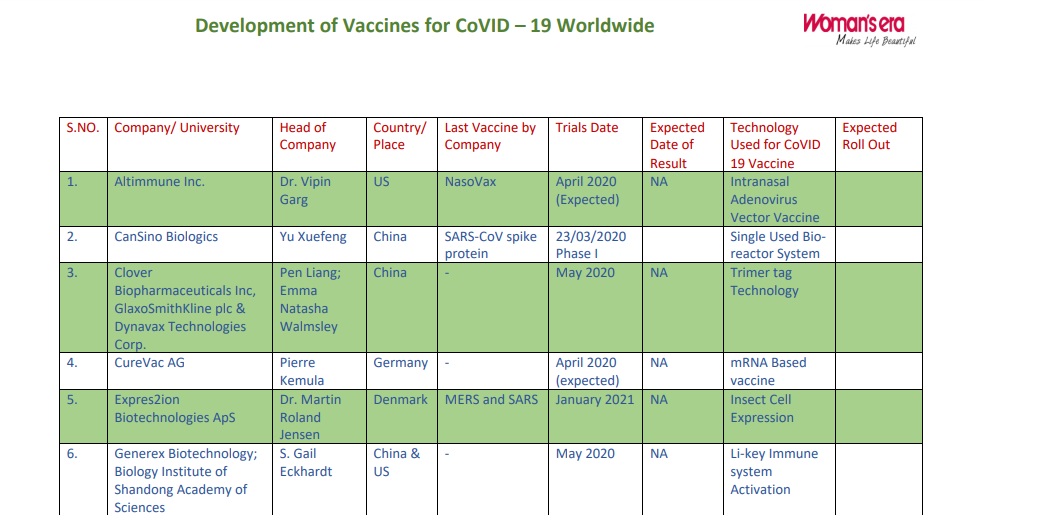
| S.NO. | Company/ University | Head of Company | Country/ Place | Last Vaccine by Company |
Trials Date | Expected Date of Result |
Technology Used for CoVID 19 Vaccine |
Expected Roll Out |
| 1. | Altimmune Inc. | Dr. Vipin Garg | US | NasoVax | April 2020 (Expected) | NA | Intranasal Adenovirus Vector Vaccine |
|
| 2. | CanSino Biologics | Yu Xuefeng | China | SARS-CoV spike protein | 23/03/2020 Phase I |
Single Used Bioreactor System | ||
| 3. | Clover Biopharmaceuticals Inc, GlaxoSmithKline plc &Dynavax Technologies Corp. |
Pen Liang; Emma Natasha Walmsley and CEPI |
China | – | May 2020 | NA | Trimer tag Technology | |
| 4. | CureVac AG | Pierre Kemula and CEPI |
Germany | – | April 2020 (expected) | NA | mRNA Based vaccine | |
| 5. | Expres2ion Biotechnologies ApS |
Dr. Martin Roland Jensen |
Denmark | MERS and SARS | January 2021 | NA | Insect Cell Expression | |
| 6. | Generex Biotechnology; Biology Institute of Shandong Academy of Sciences |
S. Gail Eckhardt and Melinda Foundation |
China & US | – | May 2020 | NA | Li-key Immune system Activation |
|
| 7. | IbioInc; Beijing CC Pharming Ltd. | Robert B. Kay | China | – | Approval waited for clinical trials |
NA | FastPharming Sytem (Plant Produced) |
|
| 8. | Imperial College of London |
Robin Shattock and CEPI |
UK | – | April 2020 (expected) | NA | Antibody therapy |
| S.NO. | Company/ University | Head of Company | Country/ Place | Last Vaccine by Company |
Trials Date | Expected Date of Result |
Technology Used for CoVID 19 Vaccine |
Expected Roll Out |
| 9. | Inovio Pharma; Beijing Advaccine Biotech. Co. Ltd; Genome Lifescience Inc. |
J. Joseph Kim and Funds By Bill Gates | China & US | MERS | April 2020 (Human trials expected) |
September 2020 | DNA Vaccine | |
| 10. | LinearxInc; Takis Biotech | James Hayward | New York & Italy |
April 2020 (expected) | NA | Polymerase Chain Reaction |
||
| 11. | Medicago Inc. | Dr. Bruce Clark | Canada | SARS CoV 2 gene | July 2020 (expected) | NA | Basic Antibodies response | |
| 12. | Moderna Therapeutics Inc.; Kaiser Permanente Health Research Inc.; NIAID |
Bill Gates and CEPI | US | Zika Vaccine, SARS 1 | 16/03/2020 (Human trial) | NA | mRNA based Vaccine | |
| 13. | Novavax Inc. | Staney C. Erckand CEPI |
US | MERS | May 2020 (expected) | NA | Nanoparticles vaccine | |
| 14. | The Jenner Institute; University of Oxford | Prof. Adrian Hill |
UK | MERS | 28/03/2020 (Phase I) |
NA | Adenovirus Vector Encoding |
|
| 15. | Stermirna therapeutics Co. Ltd.; Tongji University |
Li Hangwen | China | – | April 2020 (expected) | NA | mRNA based vaccine | |
| 16. | University of Queensland | Peter Hoj and CEPI | Australia | – | May 2020 (expected) | NA | Protein subunit based | |
| 17. | University of Saskatchewan | Richard Peter Stoicheff |
Canada | – | NA | NA | Different strains of coronavirus | |
| 18. | Vaxart Inc. | Wouter W. Latour | UK | – | June 2020 (expected) | NA | Recombinant protein vaccine |
| S.NO. | Company/ University | Head of Company | Country/ Place | Last Vaccine by Company |
Trials Date | Expected Date of Result |
Technology Used for CoVID 19 Vaccine |
Expected Roll Out |
| 19. | Vaxil Bio Ltd. | Ari S. Kellen | Israel | – | July 2020 (expected) | NA | Peptide vaccine based on bioinformatics | |
| 20. | Walter Reed Army Institute of Research and US Army Medical Research and Development Command |
Col. Deydre S. Teyhen | US | Ebola | July 2020 (expected) | NA | MERS research | |
| 21. | ZydusCadilla | Pankaj Patel | India | – | NA | NA | rMeasles Virus vector based | |
| 22. | Mayo Vaccine Research Group | Dr. Gregory Poland | China | – | 25/03/2020 | NA | Based on Peptide Subunit |
|
| 23. | University of Western Ontario | – | Canada | – | May 2020 (expected) | NA | Bioinformatics and genetic imaging | |
| 24. | National School of Tropical Medicine | Peter Hotez | US | SARS 1 | May 2020 (expected) | NA | Development from SARS | |
| 25. | Cambridge University | Jonathan Henley | UK | Ebola | Looking for Funds | NA | Antibodies induced enhancing | |
| 26. | State Key Laboratory for Emerging Infectious Diseases |
Xiang Zhang | Hong Kong, China |
H1N1 | April 2020 | NA | Based on Flu Vaccine | |
| 27. | BioNTech SE and Pfizer Inc. | UgurSahin | Germany | Cancer related | April 2020 (Expected) | NA | mRNA baaed | |
| 28. | CytoDyn Inc. | Nader Z. Pourhassan |
USA | HIV | April 2020 (Expected) | NA | Antibodies response based | |
| 29. | Heat Biologics Inc. | Jeffrey Wolf | USA | Immunotherapy | April 2020 (Expected) | NA | Based on Flu Vaccine | |
| S.NO. | Company/ University | Head of Company | Country/ Place | Last Vaccine by Company |
Trials Date | Expected Date of Result |
Technology Used for CoVID 19 Vaccine |
Expected Roll Out |
| 30. | Johnson & Johnson | Alex Gorsky | USA | HIV | September 2020 | NA | mRNA Based | |
| 31. | Regeneron Pharmaceuticals Inc |
Leonard Schleifer | USA | Ebola | May 2020 | NA | Peptide bond Based | |
| 32. | Sanofi | Paul Hudson | France | Rubella | April 2020 (Expected) | NA | – | |
| 33. | India’s Serum Institute | Cyrus Poonawalla |
India | Swine Fly | April 2020 (Expected) | January 2021 | Antibodies response Based | |
| 34. | Bharat Biotech | Dr. Krishna Ella |
India | RotaVac | April 2020 (Expected) | December 2020 | Nasal Vaccine | |
| 35. | GeoVax and BravoVax | Farshad Guirakhkoo |
US | HIV | NA | NA | – | |
| 36. | University of Pittsburgh | Louis Falo | US | MERS | 2 April 2020 Preclinical Trial |
NA | Based on SARS CoV 1 Vaccine |
| 1. | Remdesivir | antiviral protease inhibitor against coronaviruses |
investigational | Gilead, WHO, INSERM | China, Japan initially; expanded to multiple countries in Europe and N. America in Global Solidarity and Discovery Trials |
April (Chinese, Japanese trials) to mid2020 |
| 2. | Hydroxychloroquine or Chloroquine | antiparasitic and antirheumatic; generic made by many manufacturers | malaria, rheumatoid arthritis, lupus (International) | CEPI, WHO, INSERM | Multiple sites in China; Global Solidarity and Discovery Trials, Europe, international |
April 2020 (Chinese trials); mid2020
|
| 3. | Favipiravir | antiviral against influenza | influenza (China) | Fujifilm | China | April 2020 |
| 4. | Lopinavir/Ritonavir | antiviral, immune suppression | investigational combination; lopinavir/ritonavir approved | CEPI, WHO, UK Government, Univ. of Oxford, INSERM |
Global Solidarity and Discovery Trials, multiple countries | mid-2020 |
| 5. | Sarilumab | human monoclonal antibody against interleukin-6 receptor | rheumatoid arthritis (USA, Europe) | Regeneron- Sanofi |
Multiple countries | Spring 2020 |
| 6. | ASC-09 + Ritonavir | antiviral | combination not approved; ritonavir approved for HIV | Ascletis Pharma | Multiple sites in China | Spring 2020 |
|
|
|
|
|
|
|
|
| 7. | Tocilizumab | human monoclonal antibody against interleukin-6 receptor | immunosuppression, rheumatoid arthritis (USA, Europe) | GenentechHoffmann-La Roche |
Multiple countries | mid-2020 |