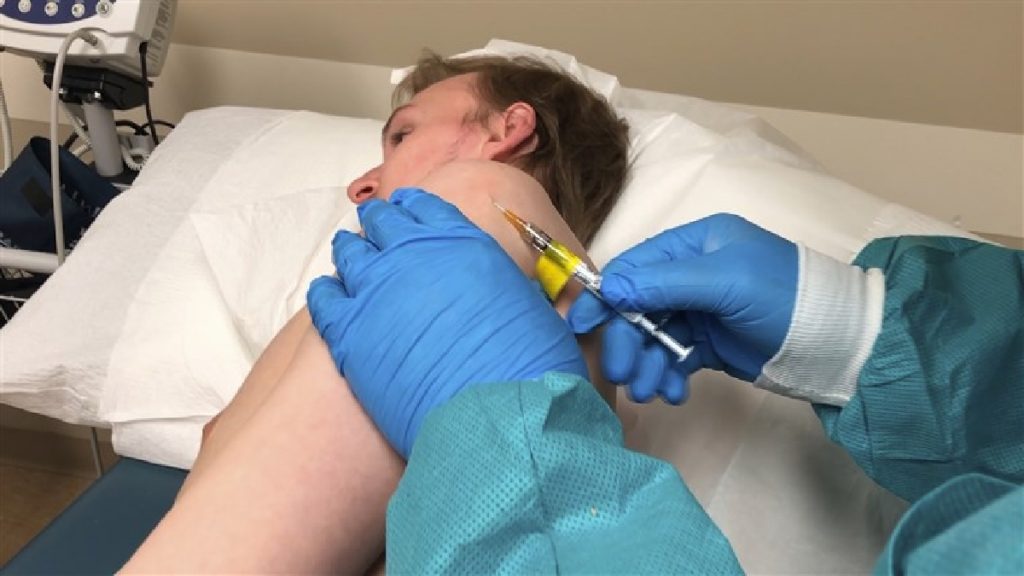
U.S. analysts have opened another security trial of an exploratory COVID-19 vaccine, this one utilizing a shallow shot rather than the standard more profound poke.
The squeeze should feel like a basic skin test, a specialist told the volunteer lying on a test table in Kansas City, Missouri, on Wednesday.
“It’s the most significant preliminary that we’ve at any point done,” Dr. John Ervin of the Center for Pharmaceutical Research told The Associated Press a short time later. “Individuals are whipping the entryway to get into this preliminary.”
The test, utilizing an antibody competitor created by Inovio Pharmaceuticals, is a piece of a worldwide chase for much-required assurance against an infection that has set off a financial shutdown and constrained individuals inside as nations attempt to stem the spread.
An alternate vaccine competitor started wellbeing testing in individuals a month ago in Seattle, one created by the U.S. National Institutes of Health. Around 66% of that review’s members have gotten the first of two required portions.
Inovio’s examination is set to test two portions of its vaccine, code-named INO-4800, in 40 solid volunteers at the Kansas City look into lab and the University of Pennsylvania. Inovio is working with Chinese scientists to likewise start a comparative report in that nation soon.
These beginning time examines are an initial step to check whether a vaccine seems safe enough for bigger tests expected to demonstrate whether it will secure. Regardless of whether the examination works out positively, it is required to take beyond what a year prior to any antibody could be broadly accessible.
Many potential vaccines are being structured in labs around the globe, expected to start this testing procedure throughout the following a while.
“The beneficial thing is we have a lot of applicants,” Dr. Anthony Fauci, the NIH’s irresistible sicknesses boss, said during a webcast for the Journal of the American Medical Association Wednesday.
The greater part of the vaccines a work in progress have a similar objective: A spike protein that studs the outside of the infection and encourages it attack human cells. However many work in very various manners, making it essential to test various choices.
Inovio analysts bundled a segment of the infection’s hereditary code inside a bit of engineered DNA. Infused as an antibody, the cells go about as a scaled down manufacturing plant to deliver innocuous protein duplicates. The safe framework makes defensive antibodies against them — prepared if the genuine infection ever goes along.
Inovio innovative work boss Kate Broderick compares it to giving the body a FBI needed banner so it can perceive the foe.
In any case, after the shallow infusion, analysts must hold a gadget over the recognize that gives a little electrical zap. The manufactured DNA is enormous with regards to infiltrating human cells, and the beat helps the antibody all the more effectively enter and get the chance to work, Broderick said.
DNA immunizations are another innovation. In any case, Inovio has trial vaccines against different illnesses that are made a similar way that have passed starting security testing.
What’s more, in any event one indicated clues that diving shallow in some way or another sped the safe framework’s advancement of defensive vaccines, University of Pennsylvania’s Dr. Pablo Tebas told The AP. Tebas drives the this most recent new COVID-19 examination.
Moderna and the National Institutes of Health beat all other players to human testing in the race for a COVID-19 vaccine. But the field is quickly growing. Aside from Moderna and Inovio, China’s CanSino Bio has one program in early human studies, and the Shenzhen Geno-Immune Medical Institute has two programs in phase 1, according to CEPI.
Inovio Pharmaceuticals initiated a Phase I trial of a DNA-based vaccine candidate called INO-4800 in collaboration with a Chinese firm and support from CEPI and the Gates Foundation.