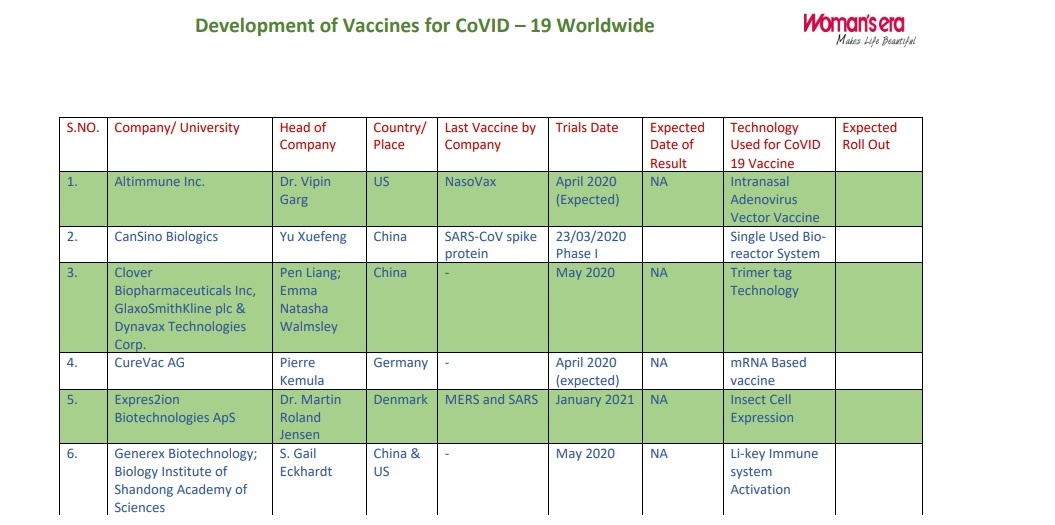
| S.NO. | Company/ University | Head of Company | Country/ Place | Last Vaccine by Company | Trials Date | Technology Used for CoVID 19 Vaccine |
| 1. | Altimmune Inc. | Dr. Vipin Garg | US | NasoVax | 30/03/2020 Phase II | Intranasal Adenovirus Vector Vaccine |
| 2. | CanSino Biologics | Yu Xuefeng | China | – | 23/03/2020 Human trial | Single Used Bioreactor System |
| 3. | Clover Biopharmaceuticals Inc, GlaxoSmithKline plc & Dynavax Technologies Corp. | Pen Liang; Emma Natasha Walmsley and CEPI | China | – | May 2020 | Trimer tag Technology |
| 4. | CureVac AG | Pierre Kemula and CEPI | Germany | – | May 2020 Clinical phase I (Expected) | mRNA Based vaccine |
| 5. | Expres2ion Biotechnologies ApS | Dr. Martin Roland Jensen | Denmark | MERS and SARS | January 2021 | Insect Cell Expression |
| 6. | Generex Biotechnology; Biology Institute of Shandong Academy of Sciences | S. Gail Eckhardt and Melinda Foundation | China & US | – | May 2020 | Li-key Immune system Activation |
| 7. | Ibio Inc; Beijing CC Pharming Ltd. | Robert B. Kay | China | – | April 2020 Pre-clinical Phase | Fast Pharming Sytem (Plant Produced) |
| 8. | Imperial College of London | Robin Shattock and CEPI | UK | – | May 2020 Human trial (Expected) | Antibody therapy |
| 9. | Inovio Pharma; Beijing Advaccine Biotech. Co. Ltd; Genome Lifescience Inc. | J. Joseph Kim and Funds By Bill Gates | China & US | MERS | 28 April 2020 Human trial Phase I | DNA Vaccine | |
| 10. | Linearx Inc; Takis Biotech | James Hayward | New York & Italy | May 2020 Pre-Clinical tests (expected) | Polymerase Chain Reaction | ||
| 11. | Medicago Inc. | Dr. Bruce Clark | Canada | SARS CoV 2 gene | July 2020 (expected) | Basic Antibodies response | |
| 12. | Moderna Therapeutics Inc.; Kaiser Permanente Health Research Inc.; NIAID | Bill Gates and CEPI | US | Zika Vaccine, SARS 1 | 16/03/2020 (Human trial) | mRNA based Vaccine | |
| 13. | Novavax Inc. | Staney C. Erck and CEPI | US | MERS | May 2020 Human Trial Phase I (expected) | Nanoparticles vaccine | |
| 14. | The Jenner Institute; University of Oxford | Prof. Adrian Hill | UK | MERS | 28/03/2020 (Phase I) | Adenovirus Vector Encoding | |
| 15. | Stermirna therapeutics Co. Ltd.; Tongji University | Li Hangwen | China | – | April 2020 (expected) | mRNA based vaccine | |
| 16. | University of Queensland | Peter Hoj and CEPI | Australia | – | May 2020 (expected) | Protein subunit based | |
| 17. | University of Saskatchewan | Richard Peter Stoicheff | Canada | – | NA | Different strains of coronavirus | |
| 18. | Vaxart Inc. | Wouter W. Latour | UK | – | June 2020 (expected) | Recombinant protein vaccine |
| 19. | Vaxil Bio Ltd. | Ari S. Kellen | Israel | – | July 2020 (expected) | Peptide vaccine based on bioinformatics | |
| 20. | Walter Reed Army Institute of Research and US Army Medical Research and Development Command | Col. Deydre S. Teyhen | US | Ebola | July 2020 (expected) | MERS research | |
| 21. | Zydus Cadilla | Pankaj Patel | India | – | NA | rMeasles Virus vector based | |
| 22. | Mayo Vaccine Research Group | Dr. Gregory Poland | China | – | 25/03/2020 | Based on Peptide Subunit | |
| 23. | University of Western Ontario | – | Canada | – | May 2020 (expected) | Bioinformatics and genetic imaging | |
| 24. | National School of Tropical Medicine | Peter Hotez | US | SARS 1 | May 2020 (expected) | Development from SARS | |
| 25. | Cambridge University | Jonathan Henley | UK | Ebola | Looking for Funds | Antibodies induced enhancing | |
| 26. | State Key Laboratory for Emerging Infectious Diseases | Xiang Zhang | Hong Kong, China | H1N1 | April 2020 | Based on Flu Vaccine | |
| 27. | BioNTech SE and Pfizer Inc. | Ugur Sahin | Germany | Cancer related | April 2020 (Expected) | mRNA baaed | |
| 28. | CytoDyn Inc. | Nader Z. Pourhassan | USA | HIV | April 2020 (Expected) | Antibodies response based | |
| 29. | Heat Biologics Inc. | Jeffrey Wolf | USA | Immunotherapy | April 2020 (Expected) | Based on Flu Vaccine | |
| 30. | Johnson & Johnson | Alex Gorsky | USA | HIV | September 2020 | mRNA Based | |
| 31. | Regeneron Pharmaceuticals Inc | Leonard Schleifer | USA | Ebola | May 2020 | Peptide bond Based | |
| 32. | Sanofi | Paul Hudson | France | Rubella | April 2020 (Expected) | – | |
| 33. | India’s Serum Institute | Cyrus Poonawalla | India | Swine Fly | April 2020 (Expected) | Antibodies response Based | |
| 34. | Bharat Biotech | Dr. Krishna Ella | India | RotaVac | April 2020 (Expected) | Nasal Vaccine | |
| 35. | GeoVax and BravoVax | Farshad Guirakhkoo | US | HIV | NA | – | |
| 36. | University of Pittsburgh | Louis Falo | US | MERS | 2 April 2020 Preclinical Trial | Based on SARS CoV 1 Vaccine |
| S.No. | Drug Candidate | Description | Trial Sponsor | Location | Expected results |
| 1. | Remdesivir | antiviral protease inhibitor against coronaviruses | Gilead, WHO, INSERM | China, Japan initially; expanded to multiple countries in Europe and N. America in Global Solidarity and Discovery Trials | April (Chinese, Japanese trials) to mid2020 |
| 2. | Hydroxychloroquine or Chloroquine | antiparasitic and antirheumatic; generic made by many manufacturers | CEPI, WHO, INSERM | Multiple sites in China; Global Solidarity and Discovery Trials, Europe, international | April 2020 (Chinese trials); mid2020 |
| 3. | Favipiravir | antiviral against influenza | Fujifilm | China | April 2020 |
| 4. | Lopinavir/Ritonavir | antiviral, immune suppression | CEPI, WHO, UK Government, Univ. of Oxford, INSERM | Global Solidarity and Discovery Trials, multiple countries | mid-2020 |
| 5. | Sarilumab | human monoclonal antibody against interleukin-6 receptor | Regeneron- Sanofi | Multiple countries | Spring 2020 |
| 6. | ASC-09 + Ritonavir | antiviral | Ascletis Pharma | Multiple sites in China | Spring 2020 |
| 7. | Tocilizumab | human monoclonal antibody against interleukin-6 receptor | GenentechHoffmann-La Roche | Multiple countries | mid-2020 |