With the Covid-19 research consortium facilitating endorsements to accelerate innovative work, general society and the private areas are concentrating on analytic units, repurposed or new medications, and immunizations against the infection that has sickened more than 800 and slaughtered 20. Pharmaceutical major, Zydus Cadila, is running pre-clinical creature preliminaries for two antibody competitors, which act totally in an unexpected way. “In another four to about a month and a half, we ought to have the consequences of the creature preliminaries.
One of the antibodies utilizes recombinant (identifying with or signifying a life form, cell, or hereditary material framed by recombination) DNA innovation and different uses switch hereditary qualities innovation (a line or expression clarifying). Contingent upon the aftereffects of the pre-clinical preliminaries, we will conclude which to take advance and apply for endorsements from the medications controller in like manner,” said Pankaj Patel, executive, Zydus Cadila.
To make antibodies through recombinant innovation, the DNA encoding an antigen that can deliver an invulnerable reaction against the infection is embedded into a bacterial or mammalian cell. Inside the cell, the antigen gets communicated and is then decontaminated to frame the immunization.
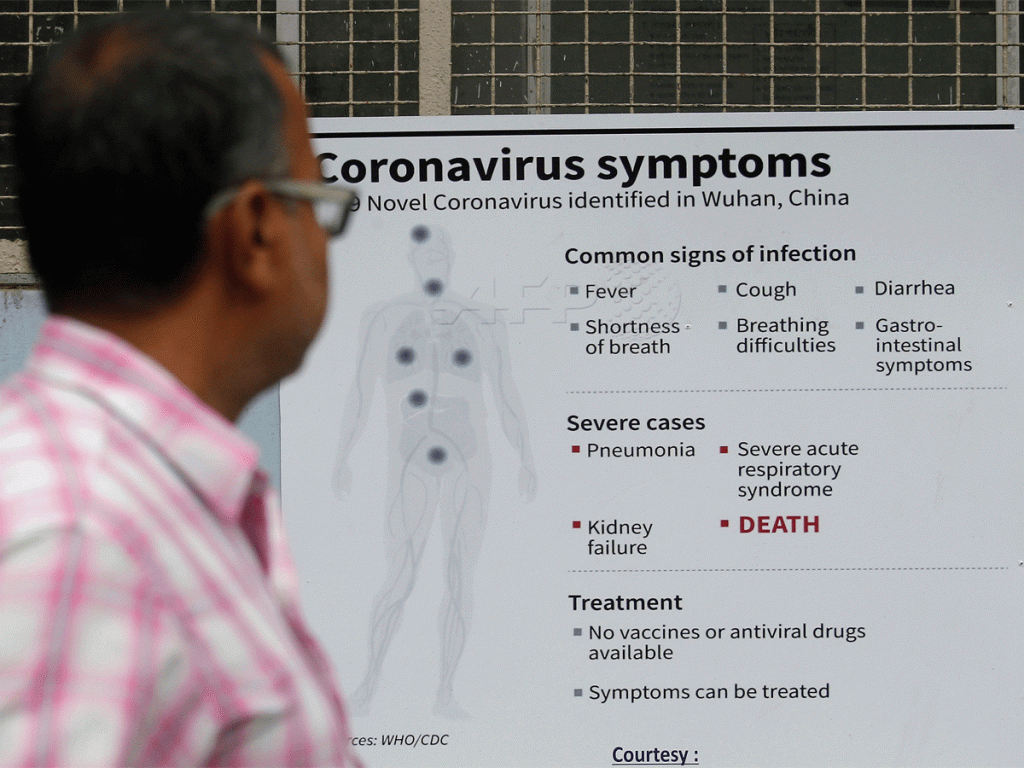
Turn around hereditary innovation can be utilized to make an infection that had debilitated intensity however is comparative enough to the coursing strain to create a safe reaction. “The immunizations are in various phases of improvement. There are a few Indian organizations and an enormous number of research bunches taking a shot at them. Be that as it may, at present, they are not seeing financing support; rather, they need support for traversing the administrative system,” said Renu Swarup, secretary, division of biotechnology.
Pune-based Serum Institute of India (SII) has begun creature preliminaries of an immunization applicant created as a team with a US biotechnology organization, Codagenix. The group plans to get the consequences of the creature preliminaries in the following two months and afterward go for endorsements for human preliminaries. All around, there are two antibodies that have started stage 1 clinical preliminaries in people and 42 others are in the pre-clinical stage, as per the World Health Organization. The two immunization applicants that are as of now in the Phase 1 preliminary have been created by the organization, CanSino Biologics, as a team with Beijing Institute of Biotechnology and Moderna, in a joint effort with the National Institute of Allergy and Infectious Diseases. Another immunization applicant created by the US organization, Inovio Pharmaceuticals, is additionally prone to go into human preliminaries in a few months.