India’s first mRNA-based Covid-19 vaccine HGCO19 has been approved by The Vaccine Expert Committee (VEC) on Tuesday. VEC has declared it as immunogenic, safe, and tolerable. It has also approved the Phase II and Phase III trials of the vaccine.
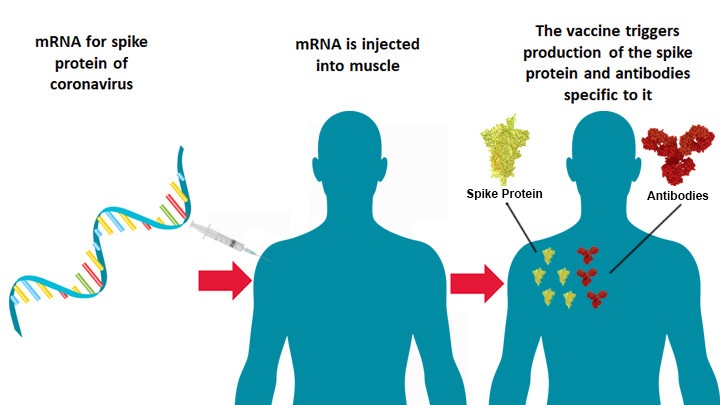
The approval came a day after the Pfozer-BioNTech vaccine (also an mRNA-based vaccine) was approved by the US Food and Drug Administration (FDA). mRNA technology uses genetic instructions for developing a vaccine to train cells of the immune system to fight against Covid-19.
The Gennova Biopharmaceuticals Ltd. is a Pune-based biotechnology company that has developed the vaccine. The interim clinical data of the Phase I trial of the vaccine was submitted for review to the Central Drugs Standard Control Organisation (CDSCO).
Dr. Sanjay Singh, CEO of Gennova Biopharmaceuticals Ltd said, “After establishing the safety of our mRNA-based COVID-19 vaccine candidate HGCO19 in Phase I clinical trial, Gennova’s focus is to start Phase II/III pivotal clinical trial. In parallel, Gennova is investing in scaling up its manufacturing capacity to cater to the nation’s vaccine requirement.”
Gennova submitted the proposed Phase II and Phase III study entitled, “A Prospective, Multicentre, Randomized, Active-controlled, Observer-blind, Phase II study seamlessly followed by a Phase III study to Evaluate the Safety, Tolerability, and Immunogenicity of the candidate HGCO19 (COVID-19 vaccine) in healthy subjects” which was approved by the office of the CDSCO.
Around 10 to 15 sites would be selected in India for conducting the study in Phase II and around 22 to 26 sites in Phase III. The use of the Indian Council of Medical Research-Department of Biotechnology (ICMR-DBT) clinical trial network sites has been planned by Gennova for conducting this study.
DBT has partly funded the development program of the mRNA-based Covid-19 vaccine in June 2020. This was further boosted under the Mission Covid Suraksha implemented by BIRAC (Biotechnology Industry Research Assistance Council), a public sector enterprise set up by the DBT.
Dr. Renu Swarup, BIRAC chairperson said, “It is a matter of great pride that Nation’s first mRNA-based vaccine is found to be safe and the Drugs Controller General of India DCGI has approved Phase II, III trials.”
Swarup said, “We are confident that this will be an important vaccine for both India and the world. This is an important milestone in our Indigenous Vaccine Development Mission and positions India on the Global Map for Novel Vaccine Development.”
About the available vaccines in India at present
Currently, 6 vaccines have got approval for emergency use in India. These include- Covaxin, Covishield, Moderna, Sputnik-V, Johnson and Johnson, and Zy-Cov-D.