US pharmaceutical giant Johnson & Johnson’s single-dose COVID-19 vaccine has received emergency use approval in India, Union Health Minister Mansukh Mandaviya tweeted.
“India expands its vaccine basket! Johnson and Johnson’s single-dose COVID-19 vaccine is approved for Emergency use in India. Now India has 5 EUA vaccines. This will further boost our nation’s collective fight against,” he tweeted on Saturday afternoon.
The shot will be brought to India through a supply agreement with homegrown vaccine maker Biological E Limited.
“We are pleased to announce that on 7th August 2021, the Government of India issued Emergency Use Authorization (EUA) for the Johnson & Johnson COVID-19 single-dose vaccine in India, to prevent COVID in individuals 18 years of age and older,” a Johnson & Johnson India spokesperson said. The healthcare major got India’s approval of its vaccine a day after it applied for Emergency Use Authorisation (EUA). The company on Friday said that Biological E will be an important part of Johnson and Johnson’s global supply chain network, helping to supply its COVID-19 vaccine Janssen through extensive collaborations and partnerships with governments, health authorities, and organizations such as Gavi and the COVAX Facility.
Studies have shown that the Johnson & Johnson vaccine is 66 percent effective in preventing cases of moderate to severe illness and 85 percent effective against severe cases of COVID-19. It completely prevented hospitalizations and death four weeks after inoculation, according to studies.
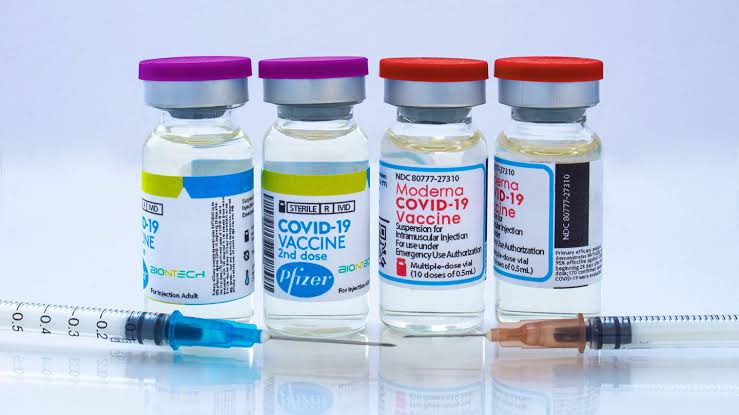
With this, India has five Covid vaccines which have been given emergency use approval. The other four are Serum Institute’s Covishield, Bharat Biotech’s Covaxin, Russia’s Sputnik V, and Moderna.
India has crossed a milestone of administering 50 crore vaccine doses under the nationwide inoculation drive. With over 49.55 lakh vaccinations in the last 24 hours, India has so far administered over 50.1 crore doses.


